Dr. Steven Mickelsen dreamed up a new way to treat a heart condition called atrial fibrillation while finishing a medical fellowship at the University of Iowa in 2012.
His idea of using pulsed electrical fields to eliminate problematic heart cells with less collateral damage than techniques that use heat or cold is at the core of Farapulse, the new medical device from Boston Scientific that earned FDA approval in January.
Though more than a decade has passed since that idea first came into his mind, the electrophysiologist, now at Scripps Health in San Diego, still has not used the technology to treat his own patients.
“For me, it has always been a conflict to be a part of the clinical trials until it’s FDA approved,” Mickelsen said. “Now I’m allowed to use it, which is exciting to be a part of something that is transformative in the field that I work in.”
Watching his colleagues use Farapulse during the trial, he said, was a bit torturous, but at the same time exhilarating.
“You know, that’s the way regulatory science has got to go; it just takes forever because even though as an inventor and investigator and doctor you might truly believe it’s a superior technology, you have to wait for the science to prove it out,” Mickelsen said. “To do a prospective randomized trial against the standard of care with a new technology, that’s just an enormous undertaking.”
Farapulse takes the same path in the body as existing technologies that are the standard of care. Surgical teams thread a long thin wire called a catheter through blood vessels, starting with a puncture in the groin and reaching the left atrium, the heart’s upper chamber that collects oxygen-rich blood from the lungs, pumping it on to feed the body.
Sometimes, cells at the back of this chamber can become damaged, causing the atrium to quiver rather than contract, which can cause dizziness and chest pain, increasing the probability of blood clots and stroke. Removal of these malfunctioning cells can significantly reduce the occurrence of this condition, which is most commonly called “afib,” and radio frequencies have been used to generate heat to eliminate cells. Extreme cold has also typically been used for this cell-removal process called ablation.
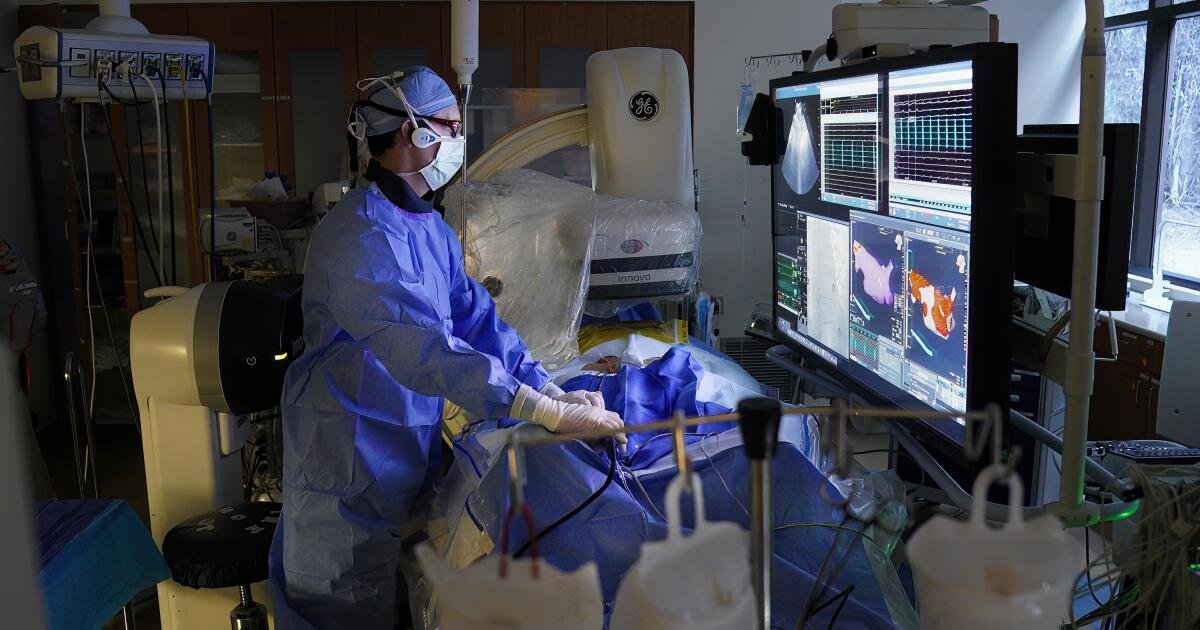
Dr. Nicholas Olson holds a pulse field ablation catheter at Scripps Memorial Hospital on Thursday.
(Nelvin C. Cepeda / The San Diego Union-Tribune )
Farapulse, and another recently approved device from Medtronic called PulseSelect, use rapid electrical signals to trigger a phenomenon called “electroporation,” which opens holes in cells walls. Heart cells are particularly susceptible to this phenomenon, but other types of tissue are unaffected. Sharp HealthCare is currently participating in yet another pulse field trial from medical device firm Biosense Webster.
Heat and cold are not so specific, notes Dr. Douglas Gibson, a Scripps electrophysiologist who was the first to use Farapulse in the San Diego region, participating in the device’s clinical trial process.
“The problem with hot and cold is they can kill anything in the body,” Gibson said. “So, we’ve had to worry about collateral damage for the past 20 years.”
For example, the esophagus passes behind the heart right in the location where ablation is conducted, and too much treatment can be disastrous.
“When you use heat it can cause an inflammatory reaction that injures the heart and the esophagus, and that reaction can cause the structures to stick together,” Gibson said.
While that reaction is uncommon — heat-based cardiac ablation has improved greatly — the ability to more easily avoid collateral damage is seen as a significant advantage. Using pulsed energy is also quicker than other methods, allowing more patients to be treated in the same amount of time. And, because the injury to tissue is less traumatic, the body does not work as hard to heal the damage, likely creating fewer chances that a second treatment will be required.

A computer model shows the treatment area for a pulse field ablation procedure at Scripps Memorial Hospital in San Diego on Thursday.
(Nelvin C. Cepeda / The San Diego Union-Tribune)
Gibson said that patients who undergo heat-based ablation often end up having flu-like symptoms after the procedure due to the inflammation that occurs after the procedure. But, in clinical trial cases, those symptoms do not appear to be common for pulse field treatment.
“On more than one occasion, we had patients say it really didn’t feel like we did much,” Gibson said.
Mickelsen said that he expects to do his first treatment with the technology he helped invent in a week or two. But he and Gibson are already working together on the next generation of pulse field technology.